- Home
- About Us
- Service
-
Products
-
- AeroTrak Remote Particle Counter with Integrated Pump 6510 & 6510-VHP
- Aerosol Sampling Manifold M32-01
- AeroTrak-Plus Remote Active Air Sampler 7010
- AeroTrak-Plus Remote Particle Counter 7510
- AeroTrak-Plus Remote Particle Counter with Integrated Pump TSI 6201
- AeroTrak-Plus Remote Particle Counter 7310
- AeroTrak-Plus Remote Particle Counter 7501
- AeroTrak-Plus Remote Particle Counter 7301
- AeroTrak-Plus Remote Particle Counter 7201
- AeroTrak-Plus Remote Particle Counter with Integrated Pump 6301
- AeroTrak-Plus Remote Particle Counter with Integrated Pump 6501
-
- VelociCalc Rotating Vane Anemometer 5725
- Alnor Velometer Thermal Anemometer AVM410, AVM420, AVM440
- Alnor Rotating Vane RVA501
- Alnor Rotating Vane RVA801
- Airflow Instruments Rotating Vanes LCA301
- Airflow Instruments Rotating Vanes LCA501
- VelociCalc Multi-Function Ventilation Meter 9565
- VelociCalc Multi-Function Ventilation Meter 9565-A
- VelociCalc® Air Velocity Meters Models 9515, 9535, 9535-A, 9545 and 9545-A
-
- DustTrak™ II Aerosol Monitor 8532
- DustTrak™ DRX Aerosol Monitor 8534
- DustTrak™ II Aerosol Monitor 8530
- DustTrak™ II Aerosol Monitor 8530EP
- DustTrak™ DRX Aerosol Monitor 8533
- DustTrak™ DRX Aerosol Monitor 8533EP
- Desktop Air Quality Monitors – M10 | M10i | M10+ / C10
- Household Portable Air Meter | S1/ S1+/ C1
- M100 – Desktop Air Quality Monitor
- Temtop M2000 2nd / M2000C 2nd – Handheld Air Quality Monitor
- Temtop LKC-1000S+ 2nd – Handheld 10-in-1 Air Quality Monitor
- Temtop P600 – Handheld Air Quality Monitor
-
- Testo 176 T4 - Temperature data logger
- Testo 176 P1 - data logger for absolute pressure, temperature and humidity
- Testo 175 H1 - Temperature and humidity data logger
- Testo 175 T2 - Two Channel Temperature data logger
- Testo 174 H - Temperature and humidity mini data logger
- Testo 184 - Temperature data logger for transport monitoring
-
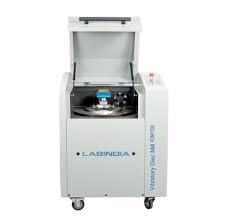
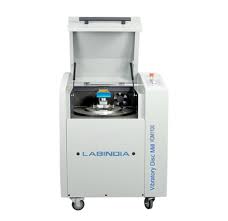
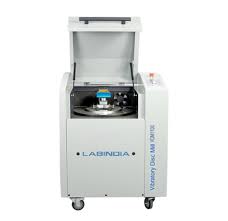
- Vibratory Disc Mill 1100
- Vibratory Disc Mill VDM 1200
- Vibratory Disc Mill VDM 1000
- High Energy Ball Mill
- Micro Ball Mill
- Cryogenic Ball Mill CM 1100
- Planetary Ball Mill BM 1100+
- Planetary Ball Mill BM 1400+
- Planetary Ball Mill BM1200+
- Hammer Mill HM 1100
- Planetary Ball Mill BM 1500+
- Planetary Ball Mill BM1150+ (Two Grinding Stations)
- Jaw Crusher JC 1000
- Pulverizer (Disc Mill)
- Automatic Pellet Press LP40T
-
- Analytical laboratory Balance-SES324C
- Analytical laboratory Balance-SES324
- Analytical laboratory Balance-SES224
- Analytical laboratory Balance-SES323
- Analytical laboratory Balance-SES423
- Analytical laboratory Balance-SES623
- Analytical laboratory Balance-SES1023
- Analytical laboratory Balance-SES1523
- Analytical laboratory Balance-SES2023
- Analytical laboratory Balance-SES3202
- Analytical laboratory Balance-SES204
- Analytical laboratory Balance-SES323S
- Analytical laboratory Balance-SES2202
- Analytical laboratory Balance-SES220C
-
- Gallery
- Partners
- Blog
- Testimonials
- Contact
- Careers





























.jpg)
.jpg)
.jpg)
.jpg)









.jpg)







.jpg)



